The ratio of the density of the hydrogen nucleus to the density of the complete hydrogen atom is approximately
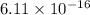 .
.
To find the ratio of the density of the hydrogen nucleus to the density of the complete hydrogen atom, let's first calculate the volumes of the nucleus and the complete atom.
The volume of a sphere is given by the formula
 , where
, where
 is the radius of the sphere.
is the radius of the sphere.
1. For the nucleus (a proton):
The radius of the proton is
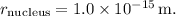
So, the volume of the nucleus is:
![\[V_{\text{nucleus}} = (4)/(3) \pi (1.0 * 10^(-15) \, \mathrm{m})^3.\]](https://img.qammunity.org/2024/formulas/physics/high-school/q7lg5ri3q5k2wwxvrjpjvmxcxr3br1ajg5.png)
2. For the complete hydrogen atom:
The radius of the electron's orbit is
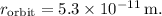
The volume of the electron's orbit can be approximated as a sphere, although the electron's path is more like an orbital cloud rather than a defined orbit. The volume is:
![\[V_{\text{orbit}} = (4)/(3) \pi (5.3 * 10^(-11) \, \mathrm{m})^3.\]](https://img.qammunity.org/2024/formulas/physics/high-school/yr9ux2mzjuvjyyuecnpkixq6r6hjzb9s5a.png)
The density of an object is given by the ratio of its mass to its volume. Since we're comparing densities, we'll assume the masses are proportional to the volumes.
The ratio of the densities of the nucleus to the complete hydrogen atom is given by:
![\[\text{Ratio} = \frac{\text{Density of nucleus}}{\text{Density of complete atom}} = \frac{V_{\text{nucleus}}}{V_{\text{orbit}}}\]](https://img.qammunity.org/2024/formulas/physics/high-school/sla9cn6v31ky8edwbgxnsmoee79f98z7q2.png)
Let's calculate this ratio:
![\[V_{\text{nucleus}} = (4)/(3) \pi (1.0 * 10^(-15) \, \mathrm{m})^3 \approx 4.19 * 10^(-45) \, \mathrm{m}^3.\]](https://img.qammunity.org/2024/formulas/physics/high-school/2tobr6q9k9jgt2mxf8k7324l1ibneb5dhs.png)
![\[V_{\text{orbit}} = (4)/(3) \pi (5.3 * 10^(-11) \, \mathrm{m})^3 \approx 6.86 * 10^(-30) \, \mathrm{m}^3.\]](https://img.qammunity.org/2024/formulas/physics/high-school/85j0tzxmby1hdnnl0qawyqg1a6n9w82kdf.png)
Now, compute the ratio:
![\[\text{Ratio} = \frac{4.19 * 10^(-45) \, \mathrm{m}^3}{6.86 * 10^(-30) \, \mathrm{m}^3} \approx 6.11 * 10^(-16).\]](https://img.qammunity.org/2024/formulas/physics/high-school/kqoxfq9zta7rh4tkqusif05cl1xmhuys3a.png)
Therefore, The answer is approximately
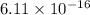 .
.