Answer: 147 mL
Step-by-step explanation:
Given:
Molarity of the sodium bromide (NaBr) solution (M1) = 1.75 M
Volume of the solution (V1) = 84 mL
Molarity of the diluted NaBr solution (M2) = 1 M
Using the dilution formula to solve for V2:
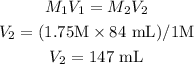
Therefore, the new volume of the solution is 147 mL