Answer:
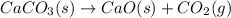
Step-by-step explanation:
Hello!
In this case, when solid calcium carbonate, CaCO3 (s), is decomposed by the action of thermal energy (heat), solid calcium oxide, CaO (s) and carbon dioxide gas, CO2 (g) are yielded via the following reaction:

However, since calcium carbonate is solid as well as calcium oxide and carbon dioxide is given off as a gas, we write:
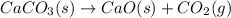
Which also balanced.
Best regards!