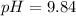Complete Question
Calculate the pH during the titration of 20.00 mL of 0.1000 M trimethylamine, (CH3)3N(aq), with 0.2000 M HClO4(aq) after 9.48 mL of the acid have been added.Kb of trimethylamine = 6.5 x 10-5.
Answer:
The pH is
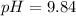
Step-by-step explanation:
From the question we are told that
The volume of trimethylamine, (CH3)3N(aq) is
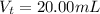
The concentration of trimethylamine is
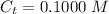
The volume of HClO4(aq) is
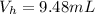
The concentration of HClO4(aq) is
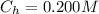
The Kb value is
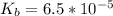
Generally the the pOH of this reaction is mathematically represented as
![pOH = pK_b + log [(N_h)/(N_b) ]](https://img.qammunity.org/2021/formulas/chemistry/college/pcp8ks667pdfww2j6jzl13ex91roxm5dv8.png)
Here
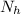 is the number of moles of acid which is evaluated as
is the number of moles of acid which is evaluated as
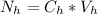
=>
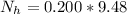
=>
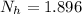
Here
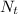 is the number of moles of acid which is evaluated as
is the number of moles of acid which is evaluated as

=>
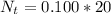
=>
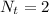
So
![pOH = -log(K_b) + log [(N_h)/(N_b) ]](https://img.qammunity.org/2021/formulas/chemistry/college/5lgem6jtb9hjn1me8jr1hwxxsrp48ahkwg.png)
![pOH = -log(6.5*10^(-5)) + log [(1.896)/(2) ]](https://img.qammunity.org/2021/formulas/chemistry/college/g93y0cv5i686o9kn1yfdr130jtta6skgc0.png)
=>
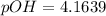
Generally the pH is mathematically represented as
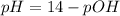
=>
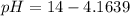
=>