Answer:
P2 = 128,205 pascal.
Step-by-step explanation:
Given the following data;
Original Pressure, P1 = 100,000 pascals
Original Temperature, T1 = 273K
New Temperature, T2 = 350K
To find new pressure P2, we would use Gay Lussac' law.
Gay Lussac's law states that when the volume of an ideal gas is kept constant, the pressure of the gas is directly proportional to the absolute temperature of the gas.
Mathematically, Gay Lussac's law is given by;
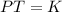
Where;
- T represents temperature.
- K is the constant of proportionality.
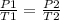
Making P2 as the subject formula, we have;
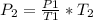
Substituting into the equation, we have;
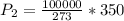
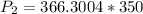
P2 = 128205.13 ≈ 128205 pascal.
Therefore, the pressure inside the hot water bottle is 128,205 pascal.