Answer: The final concentration of a solution is 0.350 M
Step-by-step explanation:
According to the dilution law,
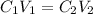
where,
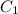 = concentration of concentrated acid solution = 12.0 M
= concentration of concentrated acid solution = 12.0 M
 = volume of concentrated acid solution = 35.0 ml
= volume of concentrated acid solution = 35.0 ml
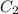 = concentration of diluted acid solution= ?
= concentration of diluted acid solution= ?
 = volume of another acid solution= 1.20 L = 1200 ml
= volume of another acid solution= 1.20 L = 1200 ml
( 1L=1000ml)
Putting in the values:
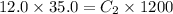
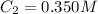
The final concentration of a solution prepared by diluting 35.0 mL of 12.0 M HCl to a final volume of 1.20 L is 0.350 M