Answer:
4.85 x 10⁻¹⁹J
Step-by-step explanation:
Given parameters:
Wavelength = 410.1nm
Unknown:
The energy of the violet light = ?
Solution:
The energy of a wave can be derived using the expression below;
E =
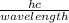
h is the planck's constant
c is the speed of light
Insert the parameters and solve;
E =
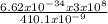 = 4.85 x 10⁻¹⁹J
= 4.85 x 10⁻¹⁹J