Answer:
650.04 mL
Step-by-step explanation:
It is given that,
The volume of the oxygen gas sample at 357 K is 765.9 mL. We need to find the volume of the sample at 303 K.
We know that, the volume is directly proportional to the temperature at constant pressure. It is Charles's law. Its mathematical form is given by :
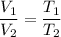
We have, V₁ = 765.9 mL, T₁ = 357 K, T₂ = 303 K, V₂ =?
Putting all the values,
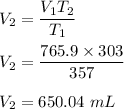
So, at 303 K, the volume is 650.04 mL.