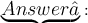
The nucleus of an atom of the element is positively charged and atom has a hollow space inside it.
Step-by-step explanation:
This experiment was conducted by Rutherford in this experiment. He placed a gold foil and surround it with a screen.
And He exposed gold foil in alpha particles rays in which he noticed that most rays passed through atom which conclude that atom has a large space, some rays get deflected and some reverse their path this show that the positive charge of nucleus of an atom.