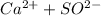Answer:
Decreasing the PH ( B )
Step-by-step explanation:
To increase the solubility of the compound CaSO4 we will need to decrease the PH level of the solution, this because the PH level of the solution CaSO4 is inversely proportional to the solubility of the solution
CaSO4 ⇄