Answer:
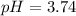
Step-by-step explanation:
Hello,
In this case, by using the Henderson-Hasselbach equation one could compute the pH considering that the pKa of hydrofluoric acid, HF, is 3.14:
![pH=pKa+log(([base])/([acid]) )](https://img.qammunity.org/2021/formulas/chemistry/college/8dmrsjloj30y2yhkiycsj9lw9h9a6w3h5m.png)
Whereas the concentration of the base and acid are computed by considering the mixing process with a total volume of 300 mL (0.3 L):
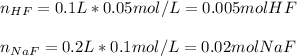
![[HF]=(0.005molHF)/(0.3L)=0.017M](https://img.qammunity.org/2021/formulas/chemistry/college/yt36q4z0zeacdzk8vu6vxnbxpocfnm3qcb.png)
![[NaF]=(0.02molHF)/(0.3L)=0.067M](https://img.qammunity.org/2021/formulas/chemistry/college/smsr8t17jtpmv0xush3zcgcga8qex5tz49.png)
Therefore, the pH turns out:
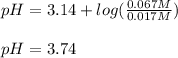
Regards.