Answer: The standard free energy change for a reaction in an electrolytic cell is always positive.
Step-by-step explanation:
Electrolytic cells use electric currents to drive a non-spontaneous reaction forward.
Relation of standard free energy change and emf of cell
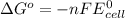
where,
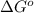 = standard free energy change
= standard free energy change
n= no of electrons gained or lost
F= faraday's constant
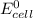 = standard emf
= standard emf
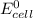 = standard emf = -ve , for non spontaneous reaction
= standard emf = -ve , for non spontaneous reaction
Thus
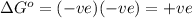
Thus standard free energy change for a reaction in an electrolytic cell is always positive.