Answer:
The ballon would be inflated. The reason is that the sodium bicarbonate in baking soda reacts with acetic acid in vinegar to produce gas.
Step-by-step explanation:
The main component of baking soda is sodium bicarbonate,
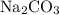 .
.
Vinegar is mostly a solution of acetic acid
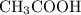 in water.
in water.
Acids such as acetic acid react with carbonate salts. One of the products of such reactions is carbon dioxide
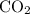 , a gas.
, a gas.
In this question, when the acetic acid in vinegar reacts with sodium bicarbonate in the baking soda, the following reaction would occur:
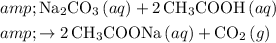 .
.
The
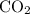 produced would then inflate the ballon placed on the opening of the bottle.
produced would then inflate the ballon placed on the opening of the bottle.