Answer:
![-(1)/(2)* (d[Br^.])/(dt)=+(d[Br_2])/(dt)](https://img.qammunity.org/2021/formulas/physics/high-school/lisg5t915mmi2x8o09vvgnjzn1bs650xjg.png)
Step-by-step explanation:
Rate of a reaction is defined as the rate of change of concentration per unit time.
Thus for reaction:
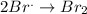
The rate in terms of reactants is given as negative as the concentration of reactants is decreasing with time whereas the rate in terms of products is given as positive as the concentration of products is increasing with time.
![Rate=-(d[Br^.])/(2dt)](https://img.qammunity.org/2021/formulas/physics/high-school/f0rwdr62ae1o64ijoyxwgte1xcy4as776f.png)
or
![Rate=+(d[Br_2])/(dt)](https://img.qammunity.org/2021/formulas/physics/high-school/7x6vkvanv72v2pjk4rzg06ljf70uhu8yp9.png)
Thus
![-(d[Br^.])/(2dt)=+(d[Br_2])/(dt)](https://img.qammunity.org/2021/formulas/physics/high-school/yd46ijv4gp9aq6on13ggnqwab4djdr4oep.png)