Answer:
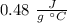
Step-by-step explanation:
In this question, we have to remember the relationship between Q (heat) and the specific heat (Cp) the change in temperature (ΔT), and the mass (m).
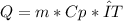
The next step is to identify what values we have:
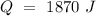
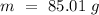
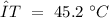
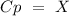
Now, we can plug the values and solve for "Cp":
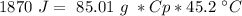
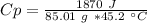
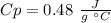
The unknow metal it has a specific value of
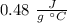
I hope it helps!