Answer: Mass of hydrogen produced is 0.0376 g.
Step-by-step explanation:
The reaction equation will be as follows.
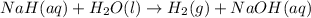
Now, formula for total pressure will be as follows.
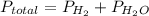
Hence,
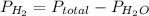
= 755 mm Hg - 42.23 mm Hg
= 712.77 mm Hg
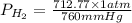
= 0.937 atm
Now, we will calculate the moles of
 as follows.
as follows.
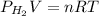
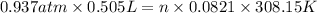
n =
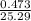 mol
mol
= 0.0187 mol
Therefore, mass of
 will be calculated as follows.
will be calculated as follows.
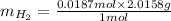
= 0.0376 g
Thus, we can conclude that mass of hydrogen produced is 0.0376 g.