Answer:
The answer is "Option A"
Step-by-step explanation:
Given:
Moles in
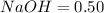
The equation formula for
 and
and
 reactions as follows:
reactions as follows:
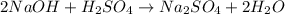
The reaction's stoichiometry:
If 2 moles of
 react with 1
react with 1
 mole
mole
Thus 0.50
 moles react with =
moles react with =
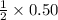
 moles
moles
So, the final value is= 0.25