Answer:
The amount of heat gained is 5225 J
Step-by-step explanation:
The specific heat of a substance is the quantity or amount of heat required to change the temperature of a unit mass of the substance by 1
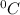 .
.
Thus,
Q = mcΔθ
where: Q is the quantity of heat required, m is the mass of the substance, c is the specific heat capacity of the substance and Δθ is the change in temperature of the substance.
From the question, given that: m = 250 g, initial temperature = 25
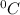 , final temperature = 30
, final temperature = 30
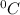 and specific heat of water = 4.18 J/g
and specific heat of water = 4.18 J/g
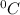 .
.
So that; the quantity of heat, Q, required is;
Q = 250 × 4.18 × (30 - 25)
= 250 × 4.18 × 5
= 5225 Joules
⇒ Q = 5225 J
Therefore, the amount of heat gained is 5225 J.