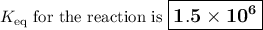Answer:
Step-by-step explanation:
We have two equations:
1. HCit + H₂O ⇌ H₃O⁺ + Cit⁻; Kₐ₁ = 8.4 × 10⁻⁴
2. NH₄⁺ + H₂O ⇌ H₃O⁺ + NH₃; Kₐ = 5.6 × 10⁻¹⁰
From these, we must devise the target equation:
3. HCit + NH₃ ⇌ NH₄⁺ + Cit⁻; Keq = ?
The target equation has HCit on the left, so you rewrite Equation 1.
4. HCit + H₂O ⇌ H₃O⁺ + Cit⁻; K₄ = Kₐ₁
Equation 4 has H₃O⁺ on the right, and that is not in the target equation.
You need an equation with H₃O⁺ on the left, so you reverse Equation 2.
When you reverse an equation, you invert its K.
5. H₃O⁺ + NH₃ ⇌ NH₄⁺ + H₂O; K₅ = 1/Kₐ
Now, you add equations 4 and 5, cancelling species that appear on opposite sides of the reaction arrows.
When you add equations, you multiply their K values.
You get the target equation 3:
4. HCit + H₂O ⇌ H₃O⁺ + Cit⁻; K₄ = Kₐ₁
5. H₃O⁺ + NH₃ ⇌ NH₄⁺ + H₂O; K₅ = 1/Kₐ
3. HCit + NH₃ ⇌ NH₄⁺ + Cit⁻; Keq = K₄K₅ = Kₐ₁/Kₐ
Keq = (8.4 × 10⁻⁴)/(5.6× 10⁻¹⁰) = 1.5 × 10⁶