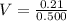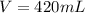Answer:
The volume of HCl required is
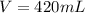
Step-by-step explanation:
From the question we are told that
The chemical equation for this reaction is
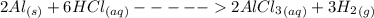
The mass of Al is
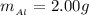
The concentration of HCl is
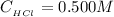
The number of moles of
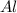 given is
given is
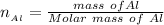
The molar mass of Al is
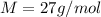
So
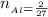
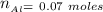
From the balanced equation
2 moles of Al reacts with 6 moles of HCl
So 0.07 moles of Al will react with x
Therefore
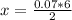
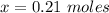
Now the volume of HCl can be obtained as
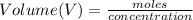
So