Answer:
D.14.7 kg
Step-by-step explanation:
Mass of block of ice=
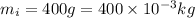
1 kg=1000 g
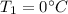
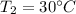
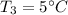
Specific heat capacities of water=
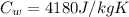
Specific heat capacities of copper=
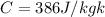
Latent heat of fusion=
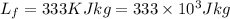
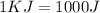
Latent heat of vaporization=
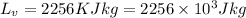
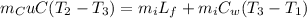
Substitute the values
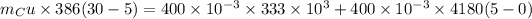
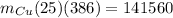
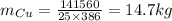
Hence, the mass of copper bowl=14.7 kg
Option D is true.