Answer:
The volume of 4.92 grams of hydrogen gas at STP is 9.105 litter.
Step-by-step explanation:
We know that, the molecular weight of hydrogen gas
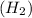 =
=
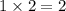 .
.
Moles of hydrogen gas =
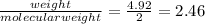 moles.
moles.
At STP, 1 mole of hydrogen gas = occupies 22.4 litter
So, 2.46 mole of hydrogen gas = occupies
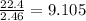 litter.
litter.
Hence, the volume of 2.46 grams of hydrogen gas at STP is 9.105 litter.