Answer:
1) Fe³⁺
2) Al³⁺
3) Pb²⁺
Step-by-step explanation:
Because all of the metal ion concentrations are the same, the order that they precipitate will be dependent on the
 values of each individual precipitate. The lower the
values of each individual precipitate. The lower the
 , the less OH⁻ needed in solution to precipitate.
, the less OH⁻ needed in solution to precipitate.
The three
 values are
values are
 = 2.6 x 10⁻³⁹ for Fe(OH)₃,
= 2.6 x 10⁻³⁹ for Fe(OH)₃,
 = 1.9 x 10⁻³³ for Al(OH)₃
= 1.9 x 10⁻³³ for Al(OH)₃
 = 1.4 x 10⁻¹⁵ for Pb(OH)₂
= 1.4 x 10⁻¹⁵ for Pb(OH)₂
This means that the ions will precipitate in the following order Fe³⁺ then Al³⁺ then Pb²⁺
Note: if too much OH⁻ is added Al³⁺ will redissolve into solution because of the formation of the soluble complex Al(OH)⁻₄ (
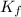 = 1.1 x 10³³)
= 1.1 x 10³³)