Answer: The pH of the resulting solution is 13.1
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
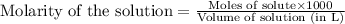 .....(1)
.....(1)
Molarity of
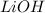 solution = 0.350 M
solution = 0.350 M
Volume of solution = 50.0 mL
Putting values in equation 1, we get:
a)
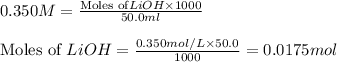
1 mole of
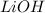 contains = 1 mol of
contains = 1 mol of

Thus
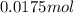 of
of
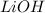 contain=
contain=
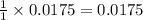 mol of
mol of

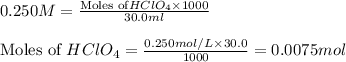
1 mole of
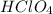 contains = 1 mol of
contains = 1 mol of

Thus
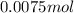 of
of
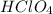 contain=
contain=
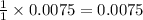 mol of
mol of


As 1 mole of
 combines with 1 mole of
combines with 1 mole of

0.0075 moles of
 combines with = 0.0075 mole of
combines with = 0.0075 mole of

Moles of
 left = (0.0175-0.0075) = 0.01
left = (0.0175-0.0075) = 0.01
Moles of
 left =
left =
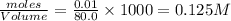
pOH =
![-log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/8yyqq29g35x7rgfeuahoap5n1go620c0bj.png)
pOH =
![-log[0.125]=0.90](https://img.qammunity.org/2021/formulas/chemistry/high-school/4b4tp7hngurhcy3ds3ipm5q5iejisyrnk7.png)
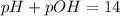
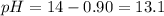
Thus the ph of the resulting solution is 13.1