Answer:
The final temperature 271.77 °C
Step-by-step explanation:
Given:
Initial pressure
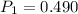 atm
atm
Final pressure
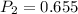 atm
atm
Initial temperature
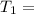 48° C = 321 K
48° C = 321 K
Initial volume
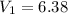 L
L
Final volume
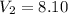 L
L
From ideal gas equation,
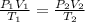
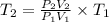
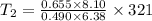
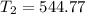 K
K
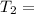 271.77°C
271.77°C
Therefore, the final temperature 271.77 °C