1.7 liters of water will be produced.
Step-by-step explanation:
The balanced chemical reaction is:
2H2+ 02⇒ 2H20
Considering the reaction to be at STP (P = 1atm, V= 22.4 L, T = 273.15 K)
the formula used is:
PV = nRT
Where P, R and T remains same only volume and number of moles are different so,
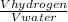 =
=
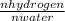
observing the balanced reaction:
mole ratio is 2:2 i.e 1:1
so volume ratio will also be same
so 1.7 litres of water will be produced.
From the reaction it is seen that
1 mole hydrogen react with oxygen to give 1 mole of water at STP.
so, it is found that 1.7 liters of hydrogen gives 1.7 liters of water