Answer:
Precipitation will occur after the concentration of fluoride ions exceeds 0.01 M.
4 mL volume of NaF must be added to cause
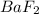 to precipitate.
to precipitate.
Step-by-step explanation:
Concentration of barium ions =
![[Ba^(2+)]=0.016 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/5nh8uqj3056sto7hr42evy36aav6bk7icz.png)
Volume of barium ion solution = 2.0 L
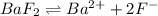
The solubility product of the barium fluoride =
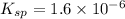
![K_(sp)=[Ba^(2+)][F^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/qqnukngc802cnoasoogezsqpimk86oleas.png)
![1.6* 10^(-6)=[0.016 M]* [F^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/2hrr69u1i130r28l1e1cpeuu3cxmznu1ey.png)
![[F^-]=0.01 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/nzdq1m4jgt6texap9djujrnpilbrvu44v7.png)
Precipitation will occur after the concentration of fluoride ions exceeds 0.01 M.
Concentration of fluoride ion =
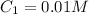
Volume of solution =
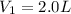
Concentration of NaF solution added =
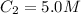
Volume of NaF solution added =
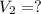
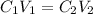
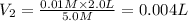
1 L = 1000 mL
0.004 L = 0.004 × 1000 mL = 4 mL
4 mL volume of NaF must be added to cause
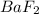 to precipitate.
to precipitate.