Answer:
(i) Cl₂ is a limiting reactant
(ii) The amount of excess reactant = 4.8 g
Step-by-step explanation:
Br₂(g) + Cl₂(g) → 2 BrCl(g) ---------------------------(i)
Calculation of no. of moles
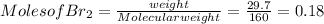
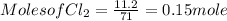
Using mole ratio method to find the limiting reactant and Excess reactant.
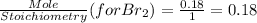
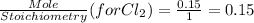
So
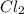 is a limiting reactant and Br₂ is excess reactant.
is a limiting reactant and Br₂ is excess reactant.
The amount of excess reactant = 0.18 - 0.15 = 0.03 mole = 4.8 g