Answer:
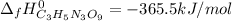
Step-by-step explanation:
Hello,
For the given reaction, the standard enthalpy of reaction is:
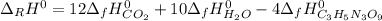
As long as the both nitrogen's and oxygen's standard enthalpies of formation are 0, thus, by looking for water's and carbon dioxide's standard enthalpies of formation, we compute the standard enthalpy of formation for nitroglycerin as shown below:
![\Delta _fH^0_(C_3H_5N_3O_9)=(1)/(4) (12\Delta _fH^0_(CO_2)+10\Delta _fH^0_(H_2O)-\Delta _RH^0)\\\Delta _fH^0_(C_3H_5N_3O_9)=(1)/(4mol) [12(-393.5kJ)+10(-241.8kJ)-(-5678kJ)]\\\Delta _fH^0_(C_3H_5N_3O_9)=-365.5kJ/mol](https://img.qammunity.org/2021/formulas/chemistry/college/gkjvq5aul8alsi5pm6s3fnpi87a4fbs3oj.png)
Best regards.