Answer:
150 joules of energy is expelled to the low temperature reservoir each minute.
Step-by-step explanation:
Lets assign the terms first.
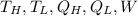 where ...
where ...
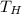 = 750 K that is, the high temperature of the reservoir.
= 750 K that is, the high temperature of the reservoir.
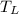 = 350 K, low temperature of the reservoir.
= 350 K, low temperature of the reservoir.
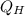 = 400 J,energy extract from the reservoir.
= 400 J,energy extract from the reservoir.
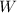 = 250 J, work done.
= 250 J, work done.
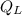 = energy expelled.
= energy expelled.
And
Formula to be used:
⇒
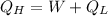
Re-arranging.
⇒
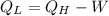
Now plugging the values of
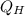 and
and
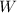 .
.
⇒
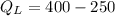
⇒
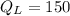 Joules
Joules
So energy expelled to the low temperature reservoir is of 150 joules.