984 grams of strontium will be recovered from 9.84x10^8 cubic meter of seawater.
Step-by-step explanation:
From the question data given is :
volume of strontium in sea water= 9.84x10^8 cubic meter
(1 cubic metre = 1000000 ml)
so 9 .84x10^8 cubic meter
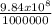 = 984 ml.
= 984 ml.
density of sea water = 1 gram/ml
from the formula mass of strontium can be calculated.
density =

mass = density x volume
mass = 1 x 984
= 984 grams of strontium will be recovered.
98400 centigram of strontium will be recovered.
Strontium is an alkaline earth metal and is highly reactive.