Answer:
Reaction follows frieldel-craft alkylation mechanism
Step-by-step explanation:
- Reaction between benzene and chloroform in the presence of
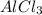 follows friedel-craft alkylation of benzene
follows friedel-craft alkylation of benzene - Firstly, one equivalent of benzene adds onto chloroform by replacing one equivalent of Cl from chloroform.
- Remaining two equivalent of Cl in chloroform are similarly replaced by two equivalent of benzene to produce triphenylmethane
- Reaction mechanism has been shown below