Answer:
We need 0.375 mol of CH3OH to prepare the solution
Step-by-step explanation:
For the problem they give us the following data:
Solution concentration 0,75 M
Mass of Solvent is 0,5Kg
knowing that the density of water is 1g / mL, we find the volume of water:
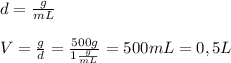
Now, find moles of
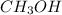 are needed using the molarity equation:
are needed using the molarity equation:
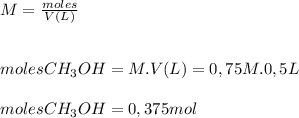
therefore the solution is prepared using 0.5 L of H2O and 0.375 moles of CH3OH, resulting in a concentration of 0,75M