Answer:
The rate of the over all reaction is ;
![R=K[A_2]^(1/2)[B]](https://img.qammunity.org/2021/formulas/chemistry/college/dqu53xlz29su2ezmllqzvteghyj6xr9j5o.png)
Step-by-step explanation:
Step 1 :
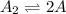 fast
fast
Step 2 :
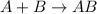 slow
slow
Equilibrium constant of the reaction in step 1:
![K_1=([A]^2)/([A_2])](https://img.qammunity.org/2021/formulas/chemistry/college/ggrmsapb5abvg9xa7wteczshwkeubh0v24.png) ....[1]
....[1]
Overall reaction:
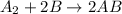
When there is a chemical reaction which taking place in more than 1 step than the rate of the over all reaction is determined by the slowest step occurring during that process;
Here step 2 is slow step, so the rate of the reaction will be;
![R=k[A][B]](https://img.qammunity.org/2021/formulas/chemistry/college/qii3elemfz2gffhxh3olepje7kd6683on9.png) ..[2]
..[2]
Putting value of [A] from [1] in [2]:
![R=k* √(K_1* [A_2])* [B]](https://img.qammunity.org/2021/formulas/chemistry/college/uy99s62njtoo7jkmz1w3gyip1qf502j8f6.png)
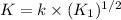
K = rate constant of the reaction
The rate of the over all reaction is ;
![R=K[A_2]^(1/2)[B]](https://img.qammunity.org/2021/formulas/chemistry/college/dqu53xlz29su2ezmllqzvteghyj6xr9j5o.png)