Answer:
The new concentration of
 will be 0.9 M.
will be 0.9 M.
Step-by-step explanation:
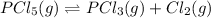
The equilibrium concentration of all species:
![[PCl_5]=0.40 M](https://img.qammunity.org/2021/formulas/chemistry/college/klp3wv6xk7mine5lvfdif55gq0v7u4udwr.png)
![[PCl_3]=[Cl_2]=0.20 M](https://img.qammunity.org/2021/formulas/chemistry/college/k8t7xlno40lq8wpq2si6fb8xn3b4r2mdwi.png)
The equilibrium constant's expression can be written as:
![K_c=([PCl_3][Cl_2])/([PCl_5])](https://img.qammunity.org/2021/formulas/chemistry/high-school/7kc3ykrhi4n2wnacy2qx27jjvxou8mgh41.png)
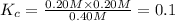
On halving the volume of the container, the concentration will get doubled;
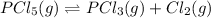
0.80 M 0.40M 0.40 M
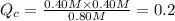
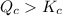
Reaction will go backward.
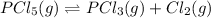
Initially
0.80 M 0.40M 0.40 M
After reestablishment of an equilibrium
(0.80+x) M (0.40-x)M (0.40-x) M
So, the equilibrium expression for above reaction can be written as :
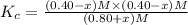
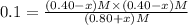
x = 0.1 M
The new concentration of
 will be:
will be:
![[PCl_5]=(0.80+x) M=(0.80+0.1) M = 0.90](https://img.qammunity.org/2021/formulas/chemistry/college/da21tvnoapaarfq4oe1e4e9nvtq213cf3e.png)