The question is incomplete, complete question is ;
While idly tossing some keys from hand to hand one day, your friend Reuben (an expert chemist) says this: "Soluble metal oxides form hydroxides when dissolved in water." Using Reuben's statement, and what you already know about chemistry, predict the products of the following reaction. Be sure your chemical equation is balanced.
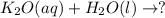
Answer:
The product will be potassium hydroxide.
Step-by-step explanation:
When aqueous potassium oxide reacts with water it gives aqueous solution of potassium hydroxide as a product. And potassium hydroxide is a hydroxide of potassium metal with formula KOH.
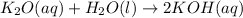
According to recation , 1 mole of potassium oxide when recats with 1 mole of water to give 2 moles of potassium hydroxide.