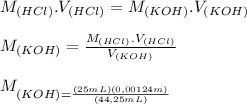Answer:
The Molarity of KOH is
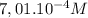
Step-by-step explanation:
The endpoint indicates the volume necessary to neutralize the moles of acid.
In other words, the point at which the moles of both solutions are the same.
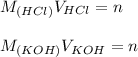
we match these equations and find the concentration of KOH