Answer:
The room temperature will be
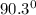
Step-by-step explanation:
We know from First law of thermodynamics that the amount of heat flow (Q) through a system is given by
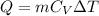
where, 'm' is the mass of the system,
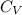 is the specific heat and
is the specific heat and
 is the temperature difference. Also we know,
is the temperature difference. Also we know,
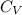 = 0.718 KJ
= 0.718 KJ
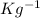 .
.
According to the problem, the heat flow can also be written as
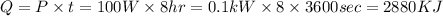
So,
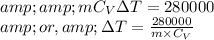
Again, if 'm' be the mass of the gas and 'R' be the Universal gas constant =
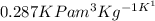 then from Ideal gas equation we can write,
then from Ideal gas equation we can write,
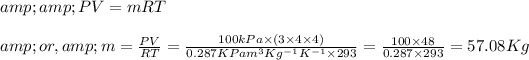
So,
