Answer
The empirical formula is CrO₂Cl₂
Step-by-step explanation:
Empirical formula is the simplest whole number ratio of an atom present in a compound.
The compound contain, Chromium=33.6%
Chlorine=45.8%
Oxygen=20.6%
And the molar mass of Chromium(Cr)=51.996 g mol.
Chlorine containing molar mass (Cl)= 35.45 g mol.
Oxygen containing molar mass (O)=15.999 g mol.
Step-1
Then,we will get,
Cr=
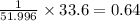 mol
mol
Cl=
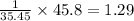 mol.
mol.
O=
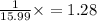 mol.
mol.
Step-2
Divide the mole value with the smallest number of mole, we will get,
Cr=
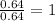
Cl=
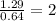
O=
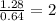
Then, the empirical formula of the compound is CrO₂Cl₂ (Chromyl chloride)