Answer: The molarity and molality of styrene solution is 0.58 M and 0.66 m respectively
Step-by-step explanation:
To calculate the mass of solvent, we use the equation:
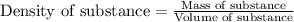
Density of solvent = 0.88 g/mL
Volume of solvent = 250. mL
Putting values in above equation, we get:
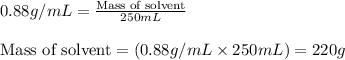
- Calculating the molarity of solution:
To calculate the molarity of solution, we use the equation:
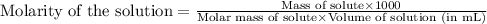
Given mass of styrene = 15. g
Molar mass of styrene = 104.15 g/mol
Volume of solution (solvent) = 250. mL
Putting values in above equation, we get:
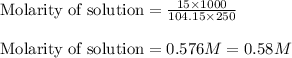
- Calculating the molality of solution:
To calculate the molality of solution, we use the equation:
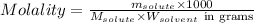
Where,
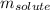 = Given mass of solute (styrene) = 15 g
= Given mass of solute (styrene) = 15 g
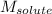 = Molar mass of solute (styrene) = 104.15 g/mol
= Molar mass of solute (styrene) = 104.15 g/mol
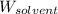 = Mass of solvent = 220 g
= Mass of solvent = 220 g
Putting values in above equation, we get:
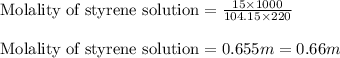
Hence, the molarity and molality of styrene solution is 0.58 M and 0.66 m respectively.