Answer:
246.67 min
Step-by-step explanation:
Half life is the time at which the concentration of the reactant reduced to half.
Half life expression for first order kinetic is:
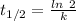
Where, k is rate constant
So,
Given that;- k =
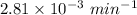 (Values corrected from source)
(Values corrected from source)
Thus,
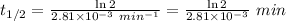
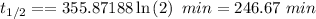
246.67 min is the half-life of the reaction.