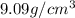The question is incomplete, here is the complete question:
Solid cesium iodide has the same kind of crystal structure as CsCl which is pictured below: If the edge length of the unit cell is 456.2 pm, what is the density of CsI in
The image is attached below.
Answer: The density of CsI is
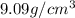
Step-by-step explanation:
To calculate the density of metal, we use the equation:
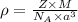
where,
 = density
= density
Z = number of atom in unit cell = 2 (BCC)
M = atomic mass of CsI = 259.8 g/mol
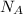 = Avogadro's number =
= Avogadro's number =
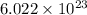
a = edge length of unit cell =
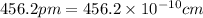 (Conversion factor:
(Conversion factor:
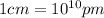 )
)
Putting values in above equation, we get:
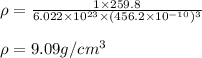
Hence, the density of CsI is