Answer:
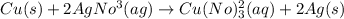
Step-by-step explanation:
The given reaction has been occurred when the copper being more reactive than that of the silver. Which will subsequently displace the silver cations present in it, (by reducing it to the elemental metal). Copper being itself will get oxidized to the copper (II) cation forming
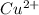 and dissolve in the solution. So, the final product formed with the silver metal will start precipitation out and giving the copper (II) nitrate as resultant in the solution.
and dissolve in the solution. So, the final product formed with the silver metal will start precipitation out and giving the copper (II) nitrate as resultant in the solution.