Answer: 459 nm
Step-by-step explanation:
The relation between energy and wavelength of light is given by Planck's equation, which is:
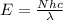
where,
E = energy of the light =
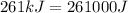 (1kJ=1000J)
(1kJ=1000J)
N= avogadro's number =
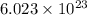
h = Planck's constant =
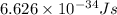
c = speed of light =
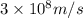
 = wavelength of light = ?
= wavelength of light = ?
Putting the values in the equation:
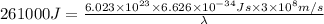
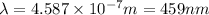
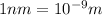
Thus the longest wavelength of light that can be used to eject electrons from the surface of this metal via the photoelectric effect is 459 nm