Step-by-step explanation:
The mass of carbon and hydrogen is calculated from the mass of their oxides (
 and
and
 ) as follows.
) as follows.
Mass of C =
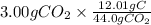
= 0.818 g C
Mass of H =
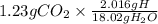
= 0.137 g H
So, the mass of C + mass of H is as follows.
0.818 g + 0.137 g = 0.955 g
This mass is actually less than the mass of sample. And, the missing mass must be caused by O. Hence, the mass of O will be calculated as follows.
Mass of O = 1.50 g - 0.955 g
= 0.545 g
Now, we convert masses to moles and find their moles and ratio as follows.
Element Mass/g Moles Ratio
 Integers
Integers
C 0.818 0.068 1 2 2
H 0.137 0.068 1 2 2
O 0.545 0.0340 0.5 1 1
Thus, we can conclude that simplest formula for the given compound is
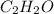 .
.