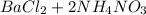Answer:
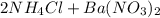 ⇒
⇒
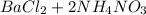
Step-by-step explanation:
In balancing a chemical equation we make sure the number of atoms of each element on the reactant side of the equation equals that on the product side
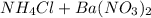 ⇒
⇒
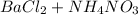
The equation above represents an unbalanced equation of the reaction of aqueous ammonium chloride with aqueous barium nitrate .we can balance this equation by adding the right coefficients to reactants and product.
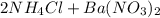 ⇒
⇒