Answer:
Q5.
a} Yes , All Cl2 get eliminated by adding 170 g of sodium thiosulfate .
b) There excess of Sodium thiosulfate by 2.637 g
Q6.
a) Mass = 162 g Sodium bicarbonate
b)Volume of Ammonia remain after the reaction = 0.086 x 24.8 = 1.60 L
Step-by-step explanation:
Points to be considered :
There is a difference between STP and SATP :
STP = Standard Temperature and Pressure (273.15 K and 1 atm)
1 mole of gas at STP = 22.4 L
SATP = Standard Ambient Temperature and Pressure (293.15 K and 1 atm)
1 mole of gas at SATP = 24.8 L
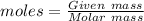
Number of moles of gas at SATP :
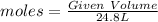
Q5.
First, calculate the number of moles of Cl2 and thiosulfate present in the reaction :
Volume of Cl2 = 105 L
Moles of Cl2 =
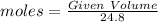
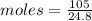
Moles of Cl2 in Reaction medium = 4.2338 mole
Mass of Sodium thiosulfate = 170 g
Molar mass of thiosulfate = 158.11 g/mol (theoretical value)
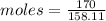
= 1.075
Moles of Sodium Thiosulfate in Reaction medium = 1.075 mole
To check whether the given moles of Cl2 and sodium thiosulfate satisfy theoretical values :
Consider the Given reaction and apply law of conservation of mass
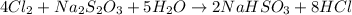
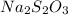 = sodium thiosulfate
= sodium thiosulfate
This equation indicates ,
4 moles of Cl2 require = 1 mole of sodium thiosulfate
1 mole of Cl2 require =
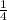 of sodium thiosulfate = 0.25
of sodium thiosulfate = 0.25
4.2338 mole of Cl2 should need = 0.25 x 4.2338
= 1.058 mole of sodium thiosulfate
Required Thiosulfate = 1.058 mole
But,
Moles of Sodium Thiosulfate in Reaction medium = 1.075 mole
So , extra moles of Sodium Thiosulfate is present in the reaction by
= 1.075 - 1.0589 = 0.0166 mol
Molar mass of sodium thiosulfate = 158 .11 g/mol
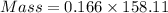
= 2.637 g
a} .Yes , All Cl2 get eliminated by adding 170 g of sodium thiosulfate .
b) There excess of Sodium thiosulfate by 2.637 g
Q6.
Volume of ammonia = 50.0 L
Moles of Ammonia ,
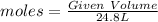
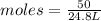
= 2.016 moles
Moles of CO2 =
Mass of CO2 = 85.0 g
Molar mass = 44 g/mol
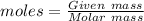
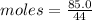
= 1.932 mol of CO2
Now check the law of conservation of mass :
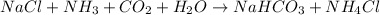
According to above equation ,
1 mole of CO2 Needs = 1 mole of NH3
1.93 mol of CO2 need = (1 x 1.93) mol
1.93 mol of CO2 need = 1.93 mol of ammonia
Available ammonia = 2.016 mol
So Ammonia is in excess by:
= 2.016 - 1.93 mol
= 0.086 mol
Volume at SATP is calculated by
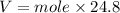
Volume of Ammonia remain after the reaction = 0.086 x 24.8
= 2.1104 L
= 2.10 L
CO2 is the limiting reagent and governs the product formation :
Molar mass of NaHCO3 = 84.007 g/mol
1 mole of CO2 Needs = 1 mole of NaHCO3 = 84.007
1.93 mol of CO2 need = 1.93 x 84.007 mol
= 162.133 g of Sodium bicarbonate
= 162 g Sodium bicarbonate
Note : The answers are present in rounded figures .