How many calories would be needed to evaporate half of a 300kg puddle of water?
90,000,000 Calories would be needed to evaporate half of a 300kg puddle of water.
Step-by-step explanation:
On average, 1 liter of water has a mass of 1 kg which requires 540,000 calories or
 Joules of energy to get evaporated when the temperature rises to 100 degrees. In the event that the water starts at a temperature T degrees, at that point an extra
Joules of energy to get evaporated when the temperature rises to 100 degrees. In the event that the water starts at a temperature T degrees, at that point an extra
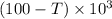 calories would be required to raise.
calories would be required to raise.
Since for 300kg of water we need 162000000 calories of energy .
Given:
1 kg = 540,000 calories or

300Kg =
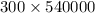
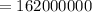
If half of the 300kg puddle has evaporate. Then
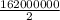
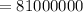 calories of energy
calories of energy
So the evaporate value of 300kg puddle of water will be around 90000000 calories.