The molar mass of
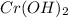 is 86.02 g/mole .
is 86.02 g/mole .
Explanation:
The molar mass of a chemical compound is represented as the mass of a unit of that compound separated by the number of substances in that unit, measured in moles. The molar mass is a volume, not molecular, the property of a substance.
The molar mass is a percentage of various examples of the compound, which usually change in mass due to the appearance of isotopes.
From the below attached table, the Molar mass of
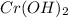 is 86.0108 g/mol.
is 86.0108 g/mol.