Answer:
186.357 grams
Step-by-step explanation:
Convert grams of
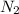 to moles:
to moles:
145g
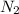 x ( 1 mole
x ( 1 mole
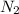 ÷ 28.02 g
÷ 28.02 g
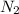 ) = 5.174 moles
) = 5.174 moles
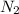
multiply the mole ration of NO by
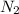 :
:
5.174 moles
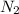 x ( 6 moles NO ÷ 5 moles
x ( 6 moles NO ÷ 5 moles
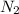 ) = 6.209 moles NO
) = 6.209 moles NO
now convert moles to grams:
6.209 moles NO x ( 30.01g NO ÷ 1 mole NO) = 186.357 grams NO
molar mass of
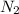 is 28.02g/mole
is 28.02g/mole
molar mass of NO is 30.01g/mole